Since ages, anaesthetists have been using the intrathecal (IT) space to provide optimum anaesthesia and analgesia. Especially, to help patients bear the pain during the operative period, and also for postoperative pain relief. Cancer pain can arise due to a tumor compressing or infiltrating tissue. In addition to this, pain occurs in 67% of the patients with metastatic cancer. Treatments such as chemotherapy, radiations, and surgery might give rise to painful conditions that continue for a longer duration, even after the treatment is completed. Hence, the method using intrathecal drug delivery system is been considered more beneficial and efficient as compared to the other methods.
Benefits of Intrathecal drug delivery system
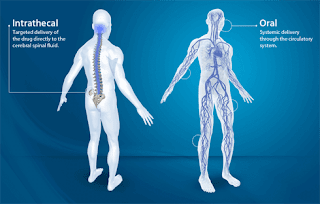
Intrathecal drug delivery treatment is mainly used to give more comfort and relief from chronic pain so that the patient bears the pain and gets cured easily. Some of these drugs have been able to lessen or even eradicate oral or systemic pain medications. An intrathecal drug delivery system consists of a catheter connected to a pump. The pump can be an external system or a completely implanted device with a container that can be refilled percutaneously. The fully implantable pumps come in two different types: one is the fixed-rate and the other is programmable. Also, various infusion options are available in these pumps such as simple continuous or more complex variable rates.
Intrathecal drug delivery systems are not a complete cure and may not help the patient get rid of all the pain or spasm—patients will still have to continue with their other medicines. However, it is an ideal treatment for controlling the symptoms of the current medicines and can help patients be more active, return to their everyday activities, and sleep better.
A potential research has stated that considerable improvements have been observed in the mood, pain, and function of the patients implanted with an Intrathecal drug delivery system. However, patients who were not treated through an IT device exhibited a significant deterioration of the physical functions, depression, and anxiety scores.
Growing demand for Intrathecal drug delivery devices
The major factors accountable for the growth in development of these systems are the growing demand for intrathecal drug delivery system for treating cancer pain. Furthermore, rise in the use of cost-effective cancer pain treatment using such systems is anticipated to fuel the growth of intrathecal drug delivery system sector in the upcoming years. However, some mechanical complications in the ITDD systems are limiting the growth of the intrathecal drug delivery systems market. Some of these mechanical complications include a variety of errors that can lead to a sudden drop in medication administration resulting in potential withdrawal, or a considerable overdose resulting in respiratory depression, hemodynamic instability, and possibly death.
Rising investments of market players in Intrathecal drug delivery system market
Several major companies in Intrathecal drug Delivery Systems are profoundly investing in IT enabled technological up-gradation of Intrathecal drug delivery systems that are programmable as well as able to control the flow of drug into the patient’s body. Additionally, some of these companies are making efforts for getting FDA approval for their products and are participating in activities such as partnerships and collaborations. For instance, Flowonix Medical, Inc., a medical device company and Cerebral Therapeutics, Inc., a clinical-stage pharmaceutical company declared the successful implant of the fifth patient in the ADDRESS (Australian Direct Drug Administration for Refractory Epilepsy) trial. Together, they have established a treatment consisting of a micro-infusion device that manages the drug delivery to a part of the brain for patients having medically refractory epilepsy.
The growing investments by these companies in research & development activities, to develop an effective and advance pain management solution is boosting the growth of Intrathecal Drug delivery systems market all across the world. For instance, in the U.S, Medtronic plc declared the approval of the U.S. Food and Drug Administration (FDA) for its new SynchroMed II myPTM Personal Therapy Manager for patients with chronic pain. This device helps patients ease their unpredictable pain by offering boluses as per requirement, or drug doses within therapeutic limits prescribed by their doctor.
Medtronic plc is one of the well-known manufacturers of fully programmable intrathecal drug delivery systems. The other major players in the industry include Teleflex Incorporated, Smiths Group Plc, Flownix Medical Inc, DePay Synthes, Tricumed Medizintechnik GmbH, Summit Medical Group, B Braun Melsungen AG, Becton, Dickinson & Company, and Fresenius Medical Care AG & Co. KGaA.
Apart from these companies, Alcyone Lifesciences is one of the companies that has recently received FDA's breakthrough designation for its delivery system for intrathecal bolus administration of medicines to cure severe central nervous system disorders. The ThecaFlex DRx system has a port that is implanted below the patient’s skin and an intrathecal catheter for cerebrospinal fluid aspiration as well as infusion of treatments.
Future of Intrathecal drug delivery system market
Since the last two decades, IT therapy has been gaining acceptance as an alternative standard medical treatment for cancer patients with medium to severe intractable pain. In North America, the development of fully implanted Intrathecal drug system is increasing day by day owing to its easy and comfortable usage with better control and long-lasting nature of these Intrathecal devices. Moreover, according to a research report by Research Dive, the Intrathecal drug delivery system market is expected to generate a revenue of $1,662.0 million by the end of 2017 and will grow at a CAGR of 6.9% from 2020 to 2027. Hence, it is clear that the growing acceptance of Intrathecal drug delivery system, rising investments in the development of Intrathecal drug delivery devices, and advanced benefits of this device are sure to boost the growth of the market significantly, in the upcoming years.
Comments
Post a Comment